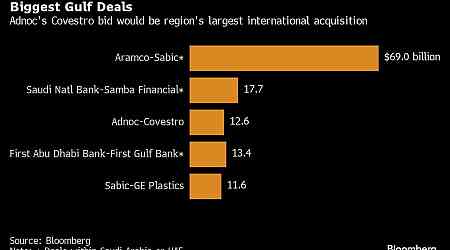
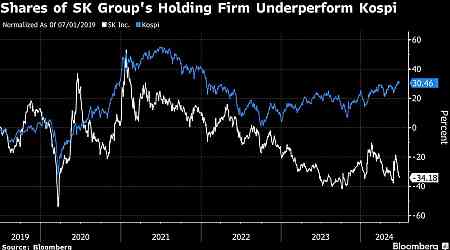
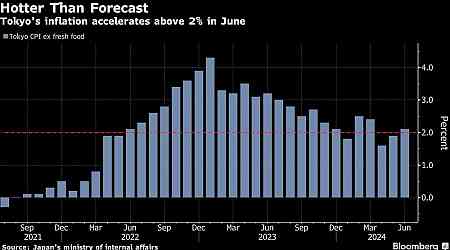
Financial Personal
Takeda Presents Late-Breaking Data from Phase 2b Study of Mezagitamab, Demonstrating Potential to Transform Treatment of Primary Immune Thrombocytopenia
Mezagitamab-Treated Patients Showed Rapid and Sustained Increases in Platelet Counts That Persisted 8 Weeks After the Last Dose Through to Week 161 Mezagitamab Had a Favorable Safety Profile, with No New Safety Signals1 Takeda Plans to Initiate Globa
OSAKA, Japan & CAMBRIDGE, Mass. -- Takeda ( TSE:4502/NYSE:TAK) today presented positive results from its Phase 2b, randomized, double-blind, placebo-controlled study evaluating the safety, tolerability and efficacy of mezagitamab (TAK-079) in patients with persistent or chronic primary immune thrombocytopenia (ITP), a rare immune-mediated bleeding disorder. ITP is characterized by the accelerated destruction of platelets in blood, resulting in a decreased platelet count and an increase of bleeding that can be debilitating. These data (Abstract #LB 01.1) were presented at the oral Late-Breakthrough Session at the 32nd Congress of the International Society on Thrombosis and Haemostasis (ISTH) in Bangkok, Thailand. Takeda plans to initiate a global Phase 3 trial of mezagitamab in patients with ITP in the second half of FY2024. Read More
Related
Share this page
Guest Posts by Easy Branches